Alkanes
Definition: Alkanes are hydrocarbons that are made up solely of carbon and hydrogen attached together by single bonds only.
Alkanes are the most simple family of hydrocarbons.
| Molecular formula | Structure | Name |
|---|---|---|
| CH4 | 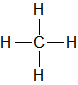
| Methane |
| C2H6 |
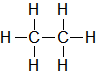 | Ethane |
| C3H8 |
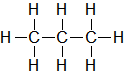 | Propane |
| C4H10 |
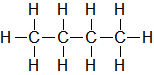 | Butane |
The alkanes are:
- Composed of carbon and hydrogen only.
- One or more carbon atoms held together by single bonds. All of the other available spaces around each carbon atom are filled with hydrogen atoms.
- The general formula for an alkane is CnH(2n+2).
- There are two main types of basic alkanes; chain and branched.
- Alkanes are also known as paraffins – this is their old name.
- Also called saturated hydrocarbons. The term refers to the fact that the each of the carbon atoms is surrounded by 4 other atoms – there is no space for more atoms to fit.
- The carbon atoms are all sp3 hybridised, which means that the neighbouring atoms are around the carbon atom in a tetrahedral shape.
- The simplest known hydrocarbon, methane, is an alkane.
- Alkanes are widely used in society:
- Around the home the simpler alkanes such as methane, propane and butane are used in cooking and other applications where controllable heat is required.
- Octane is a principle component of petrol. Other alkanes which are liquid at room temperature are found in diesel and aviation fuels.
- Solid alkanes are used in things like candles, crayons, lubrication, electrical insulation and for making roads.
- Alkanes are commonly used as the basis for other reactions. For example in making ethene and polythene.
- Primary sources of alkanes are natural deposits such as oil and gas.
- Alkanes can also be made from other hydrocarbons by either hydrogenation or substitution with hydrogen.
Physical properties
The trend in the boiling point and melting points of the first few alkanes can be seen in the table below:
| Name | Formula | Melting point (°C) | Boiling point (°C) | State at room temperature |
|---|---|---|---|---|
| Methane | CH4 | -182.5 | -161.5 | Gases |
| Ethane | C2H6 | -183.3 | -88.7 | |
| Propane | C3H8 | -187.7 | -42.1 | |
| Butane | C4H10 | -138.4 | -0.5 | |
| Pentane | C5H12 | -129.8 | 36.1 | Liquid |
| Hexane | C6H14 | -95.4 | 68.8 | |
| … | ||||
| Decane | C10H22 | -29.7 | 174.2 | |
| … | ||||
| Heptadecane | C17H36 | 22.0 | 303 | |
| Octadecane | C18H38 | 28.2 | 316 | Solid |
Table to show the melting points and boiling points of selected alkanes up to octadecane
This is summarised on the follow graph:
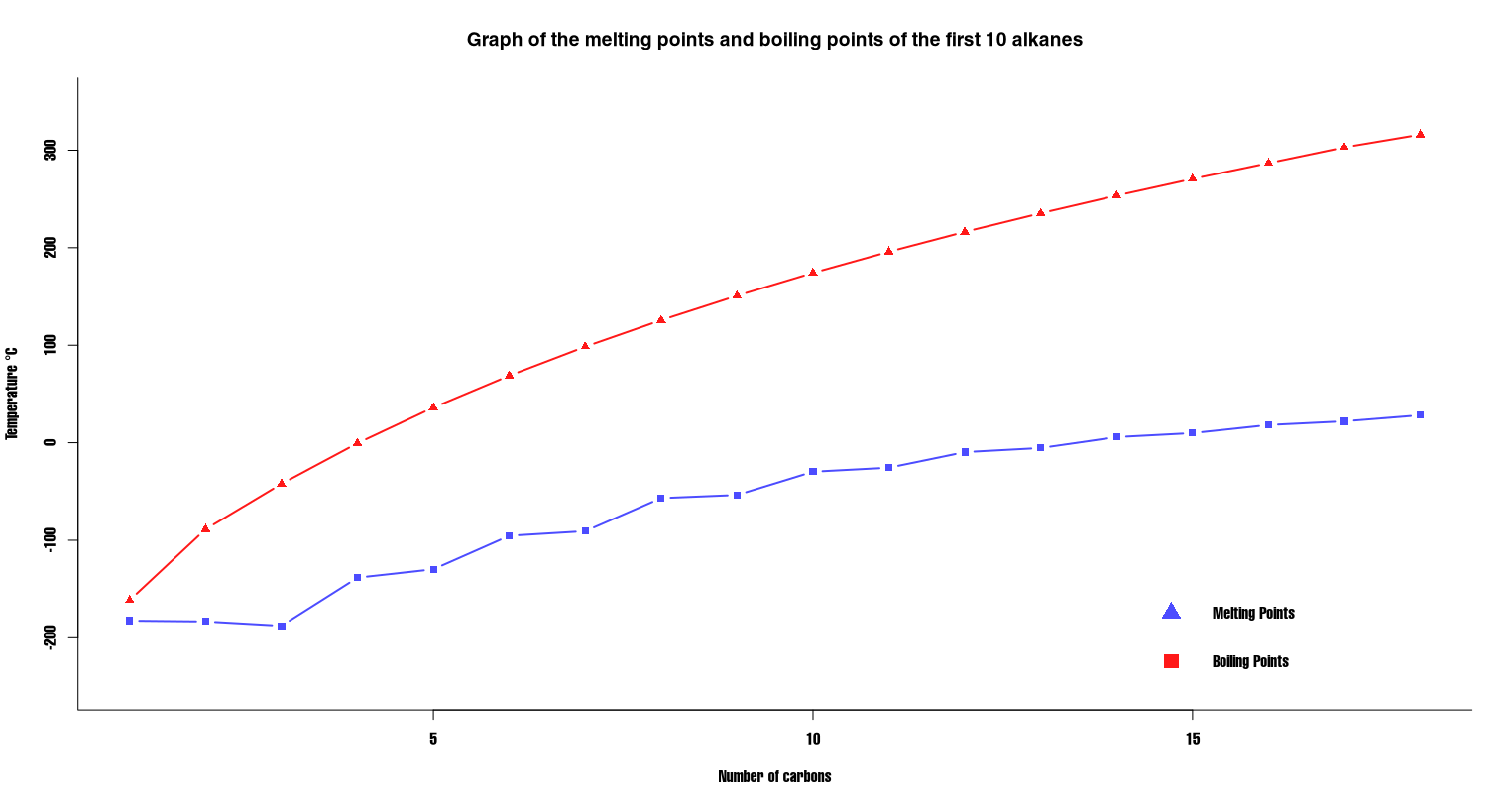
Graph of the melting and boiling points for the first 18 alkanes.
If you examine the table and graph you should be able to see the following:
- There is an upward trend in the boiling point of the alkanes as the number of carbon atoms in the alkane increases.
- There is a corresponding upward trend in the melting point, although this isn’t as well defined.
- The simplest alkanes, methane to pentane, exist as gases at room temperature (25°C) and pressure (1 atm).
- The alkanes starting at hexane and going through to heptadecane, are liquid at room temperature.
- Alkanes containing 18 (octadecane) or more carbon atoms are all solid at room temperature.
Explanation of the melting point / boiling point trend
Reminder: Boiling and melting points depend on how easy the individual molecules are to separate.
- Shorter alkanes will have less attraction between molecules (Van der Waal’s forces) because the shorter chains consist of fewer atoms. It takes less energy to separate the molecules.
- Longer chain alkanes will have much more attraction between the molecules because having more atoms allows more overlap between neighbouring atoms. More energy needs to be input to force the molecules apart.
- The alkanes are non-polar, since the electronegativities of carbon and hydrogen are similar. Therefore the intermolecular attraction does not include the stronger hydrogen-bonding (which relies on a slight charge being present on part or all of the molecules).
- Only London dispersion forces are found between alkane molecules – this is a much weaker attraction than hydrogen bonding. The difference in strength between these two bond types explains why the boiling point of methane (CH4) is low, yet the boiling point of water (H2O) is relatively high.
- The fact that alkanes possess non-polar bonds also explains why they are immiscible with water, but dissolve well with a range of organic solvents.